Regulatory Intelligence Services
Translating regulatory knowledge into action
– from “knowing” to “doing.”
We bridge the gap between understanding global life-science regulations and acting on them.
Our service rapidly gathers, compares, and structures primary source information – from FDA, EMA, ICH, PMDA, and others – into concise, decision-ready insights.
Instead of overwhelming databases or high-cost consulting, we deliver just-right, actionable intelligence focused on the specific themes that matter to your operations.

You may have read the FDA or EMA guidance.
You may even understand it.
But what exactly should your organization do next?
That’s where most teams lose time, rely on individual interpretation, and risk missing critical compliance signals.
Regulatory Signal Brief™ closes this gap.
It translates complex regulatory documents into clear operational guidance – highlighting regional differences, departmental impacts, and next-step actions across the U.S., EU, and Asia.
We provide decision-focused regulatory insights that you won’t find in conventional information services or consulting firms, available through two core plans:
Standard Reports and Custom Reports.
This report is generated from publicly available regulatory documents (ICH, FDA, EMA, etc.) using a regulatory-focused, AI-assisted analytical workflow.
This is not a generic AI summary. The workflow is designed to structure regulatory requirements and extract verification points that are often difficult to identify through standard manual review.
Final determinations must be made by your organization’s qualified experts.
Our Services
- Standard Reports(Regulatory Signal Briefs)
An 10-30 page report that organizes a specific regulatory theme. We regularly cover topics that require annual or semi-annual updates. You can purchase only the themes you need, when you need them.
- Sectors: Pharma, MedTech, SaMD, IVD, ATMP, etc.
- Regions: US, EU, Japan, Asia
- Languages: Available in English or Japanese
- Price per report: $500 USD
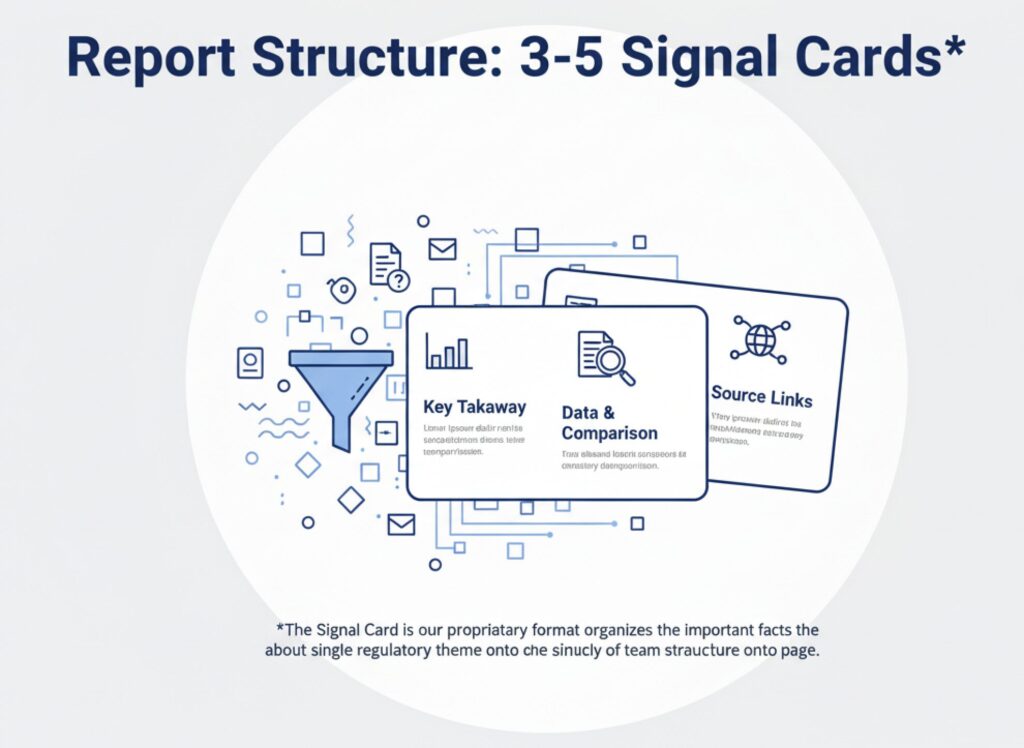
Report Structure
Executive Summary
- First ~200 characters: A clear conclusion on why this matters now and what actions are required.
- Three Signal Card highlights: The key takeaways from the selected regulatory themes.
- Top 3 immediate actions: What to implement now across teams.
Signal Cards (3–5 topics)
Each Signal Card follows the same structure:
- Card Summary: One-page overview of the theme.
- What Changed? The essential regulatory updates.
- So What? Practical impact on your organization.
- Operational Action Checklist (for discussion): Concrete steps for teams.
- Decision Prompts: The specific questions leaders must answer.
- FAQ (3 items): Common points of confusion, clarified.
- SPI (Signal Priority Index): Priority score for planning.
- Source Links: 30–40 primary URLs per card.
Business Implications Matrix
- Impact by function: Leadership / RA / QA / Clinical / IT.
- Recommended response priorities: Sequenced actions by team.
What to Watch Next
- Regulatory events to track over the next 12 months.
*What is a Signal Card?
A Signal Card is our proprietary one-page format that distills a single regulatory theme into the most important facts—change points, cross-region comparisons, and links to primary sources—always in a consistent layout. Anyone on your team can grasp the situation at a glance and use the page directly for internal discussions and reporting.
We remove noise from voluminous regulatory documents and extract only the comparisons and primary sources you need. Final interpretation and decisions remain with your experts; our purpose is to put you in a position to decide faster and with minimal effort.
Example Report Themes:
Clinical × Pharma
- Impact of the ICH E6(R3) Revision
- Comparison of DCT Guidance (FDA/EMA)
- Pediatric Study Obligations
Approval × MedTech
- EU MDR Review Delays
- Updates to the FDA Breakthrough Devices Program
- China NMPA's Accelerated Approval Programs
HTA × SaMD
- Comparison of Evaluation Criteria: Japan's DigiHTA vs. Germany's DiGA
- Trends in Digital Therapeutics Reimbursement by CMS in the US
- Custom Reports (Custom Regulatory Dossier)
We conduct bespoke research and analysis tailored to your specific products or challenges. Our custom reports bridge the gap between what internal teams cannot cover and what would otherwise require costly consulting engagements.
Example Custom Projects:
- Impact of US DCT guidance revisions on clinical trials in Japan.
- Comparison of approval pathways and accelerated programs for ADCs in the US, EU, and Japan.
- Trial design requirements for Companion Diagnostics (FDA, EMA, PMDA).
- HTA evaluation of Digital Therapeutics (DTx) in Japan, Germany, and the US.

【Are You Facing These Intelligence Dilemmas?】
The 'Information Overload' Trap
You have a contract for an expensive database, but it's full of noise, and it still takes an immense amount of time to find and understand the information that is truly important.
The 'High-Cost Project' Barrier
You just want to confirm a specific point, but requesting it from a consulting firm turns into a large-scale project where the budget and timeline don't align with your needs.

The Risk of 'Key Person Dependency' and Oversights
Your process relies on the efforts and experience of a specific team member. If that person is busy, on leave, or leaves the company, the quality of your intelligence gathering cannot be maintained, and the risk of missing critical changes increases.
【The New Alternative We Provide】
We solve these dilemmas and elevate your regulatory intelligence process to the next level.
You no longer need to drown in a flood of information.
We deliver only the "filtered signals," with the noise thoroughly removed by our curators.

You no longer need to give up due to cost or time.
We offer "agile intelligence reports" that you can purchase by the theme, delivering outstanding cost-performance.
You no longer need to rely on individual effort.
We provide a "systematic process" that eliminates key person dependency and enables anyone to obtain high-quality information.
Terms of use
- The report is provided for information purposes only and does not constitute legal or regulatory advice.
- While we make every effort to ensure the accuracy of the information,
we do not guarantee its completeness or correctness. - Unless otherwise stated, the copyright of each report belongs to Probio Corporation.
- Internal sharing within your organization is acceptable.
However, redistribution or resale to third parties without permission is not allowed. - Any decisions or actions taken based on the report are your own responsibility
(or that of your organization).
Handling of personal data
- Your information will be used only for:
- delivering the report,
- providing follow-up updates or additional related materials,
- improving and planning future reports.
- We will not share your personal data with third parties
unless required by law. - For details, please refer to our Privacy Policy on the website.
